This 2-day series of events consists of a professional COURSE DAY and a SYMPOSIUM DAY, comprising burning issues in Biosimilar development with a dedicated focus on the statistical and regulatory aspects of follow-on biologics.
During these two interactive days the forum ensures maximum knowledge transfer among professionals, regulators and leading subject matter experts in biosimilar development to exchange and elaborate on the best practices and debate the evolving landscape, pitfalls and current perspectives of biosimilars.
COURSE DAY PROGRAMME  17 OCT
17 OCT
SYMPOSIUM DAY PROGRAMME 18 OCT
18 OCT
As in previous years, visitors can take part in the event with world-prominent subject matter experts from the fields of regulatory, academic and clinical research, who will be providing a detailed overview of current development issues of follow-on biologics and guidance for their handling.
Agenda of the Symposium day
8:30 – 9:00 | 2nd day Registration
 9:00 – 10:45 | THEORETICAL CONSIDERATIONS
9:00 – 10:45 | THEORETICAL CONSIDERATIONS
- Multiplicity issues in biosimilar development programs
 Franz KÖNIG
Franz KÖNIG
Associate Professor,
Section for Medical Statistics, Medical University of Vienna, Austria
- Scientific Keynote Presentation:
Controversies on the switching and substitution of biosimilars
 László ENDRÉNYI
László ENDRÉNYI
Professor Emeritus of Pharmacology and Biostatistics, University of Toronto;
Former President of Canadian Society for Pharmaceutical Scientists, Canada
 Co-author: László TÓTHFALUSI,
Co-author: László TÓTHFALUSI,
Associate Professor,
Faculty of Pharmacology, Semmelweis Medical University, Hungary
- Balaam’s Design Revisited
 Júlia SINGER
Júlia SINGER
Chief Scientific Officer, Accelsiors CRO Ltd.;
President of the Hungarian Society for Clinical Biostatistics, Hungary
 Co-author: Joëlle MONNET GAUD
Co-author: Joëlle MONNET GAUD
Director, Biostatistics,
Fresenius-Kabi SwissBioSim GmbH, Switzerland
- Applications of the expected power (statistical assurance) for bioequivalence trials
 Arne RING
Arne RING
Professor of Statistics, University of the Free State, South Africa;
Head of Biometrics and Statistical Programming, medac GmbH, Germany
 10:45 – 11:15 | Coffee break and networking
10:45 – 11:15 | Coffee break and networking
 11:15 – 11:30 | REGULATORY QUESTIONS
11:15 – 11:30 | REGULATORY QUESTIONS
- Regulatory Keynote:
Current challenges in the development of biosimilars
 Andrea LASLOP
Andrea LASLOP
Head of Scientific Office, Austrian Medicines and Medical Devices Agency (AGES), Austria;
Member of the Scientific Advice Working Party (SAWP) of the European Medicines Agency; Austrian delegate in the Committee for Human Medicinal Products (CHMP) of the European Medicines Agency
- Quality and statistics: bringing two worlds together
 Andreas BRANDT
Andreas BRANDT
Statistical assessor,
Federal Institute for Drugs and Medical Devices (BfArM), Germany
- Regulatory reflections on biosimilar development and statistical methods used at quality level
 Ina-Christine RONDAK
Ina-Christine RONDAK
Biostatistician, Seconded National Expert from Klinikum rechts der Isar of Technische Universität München to EMA, Germany
- Is similarity different? – Recent developments from the regulatory perspective
 Stephan LEHR
Stephan LEHR
Biostatistician, Austrian Medicines and Medical Devices Agency (AGES);
Assessor, EMA Biostatistics Working Party; Alternate Member, EMA Scientific Advice Working Party;
Former President of the Viennese Section of the International Biometric Society, Austria
 13:00 – 14:00 | Lunch break and networking
13:00 – 14:00 | Lunch break and networking
 14:00 – 15:30 | CHALLENGE THE REGULATOR
14:00 – 15:30 | CHALLENGE THE REGULATOR
This extended Regulatory Panel Discussion and Q&A type of interaction replies to critical questions from the audience with a focus on the current regulatory requirements, approval process and also on the burning issues of debate facing clinical development teams specialized on biosimilars. During this discussion we will look for answers also to that how to handle differences between regulatory guideline recommendations and clinical research practice and how to adjust clinical development practice to effective legislation, or vice versa.
- Moderator:
 Stephan LEHR
Stephan LEHR
Biostatistician, Austrian Medicines and Medical Devices Agency (AGES);
Assessor, EMA Biostatistics Working Party; Alternate Member, EMA Scientific Advice Working Party;
Former President of the Viennese Section of the International Biometric Society, Austria
- Panel members:
 Andrea LASLOP
Andrea LASLOP
Head of Scientific Office, Austrian Medicines and Medical Devices Agency (AGES), Austria;
Member of the Scientific Advice Working Party (SAWP) of the European Medicines Agency; Austrian delegate in the Committee for Human Medicinal Products (CHMP) of the European Medicines Agency
 Andreas BRANDT
Andreas BRANDT
Statistical assessor,
Federal Institute for Drugs and Medical Devices (BfArM), Germany
 Ina-Christine RONDAK
Ina-Christine RONDAK
Biostatistician, Seconded National Expert from Klinikum rechts der Isar of Technische Universität München to EMA, Germany
 Ágnes GYURASICS,
Ágnes GYURASICS,
Hungarian member of EMA Committee for Human Medicinal Products (CHMP), and EMA Paediatric Committee (PDCO); Unit Head at National Institute of Pharmacy and Nutrition, Hungary
 15:30 – 16:00 | Coffee break and networking
15:30 – 16:00 | Coffee break and networking
 16:00 – 17:00 | ROUND TABLE DISCUSSION
16:00 – 17:00 | ROUND TABLE DISCUSSION
an open, structured discussion within a round table framework. This session provides an opportunity for a constructive dialogue between regulators, sponsors, academic and clinical researchers to elaborate and exchange their ideas on the current development issues an challenges of follow-on biologics.
- Moderator:
 Helmut SCHÜTZ
Helmut SCHÜTZ
Owner
BEBAC – Consultancy Services for Bioequivalence and Bioavailability Studies, Austria
- Discussants:
 Industry perspective: Heike WÖHLING
Industry perspective: Heike WÖHLING
Director Biostatistics for Biostatistics Biosimilars,
Analytics group, Novartis, Germany
 Regulatory perspective: Andrea LASLOP
Regulatory perspective: Andrea LASLOP
Head of Scientific Office, Austrian Medicines and Medical Devices Agency (AGES), Austria;
Member of the Scientific Advice Working Party (SAWP) of the European Medicines Agency; Austrian delegate in the Committee for Human Medicinal Products (CHMP) of the European Medicines Agency
- Panel members:
 Ildikó ARADI
Ildikó ARADI
Vice-Chair,
Medicines for Europe, Biosimilar Medicines Group, Hungary
 Júlia SINGER
Júlia SINGER
Chief Scientific Officer, Accelsiors CRO Ltd.;
President of the Hungarian Society for Clinical Biostatistics, Hungary
 Stephan LEHR
Stephan LEHR
Biostatistician, Austrian Medicines and Medical Devices Agency (AGES);
Assessor, EMA Biostatistics Working Party; Alternate Member, EMA Scientific Advice Working Party;
Former President of the Viennese Section of the International Biometric Society, Austria
 Franz KÖNIG
Franz KÖNIG
Associate Professor,
Section for Medical Statistics, Medical University of Vienna, Austria
 Note: The final Agenda of the 4th Annual Biosimilars Forum is under preparation. This page is frequently being updated, the detailed Agenda will be available soon here, so please come back to discover more about the programme of the event.
Note: The final Agenda of the 4th Annual Biosimilars Forum is under preparation. This page is frequently being updated, the detailed Agenda will be available soon here, so please come back to discover more about the programme of the event.
PROGRAMME OF THE 4th ANNUAL BIOSIMILARS FORUM (17-18 OCT, 2019)
This 2-day series of events consists of a professional COURSE DAY and a SYMPOSIUM DAY, comprising burning issues in Biosimilar development with a dedicated focus on the statistical and regulatory aspects of follow-on biologics.
During these two interactive days the forum ensures maximum knowledge transfer among professionals, regulators and leading subject matter experts in biosimilar development to exchange and elaborate on the best practices and debate the evolving landscape, pitfalls and current perspectives of biosimilars.
COURSE DAY PROGRAMME 17 OCT, 2019
17 OCT, 2019
SYMPOSIUM DAY PROGRAMME  18 OCT, 2019
18 OCT, 2019
Title of the short course:
- Robust methods for assessment of average and scaled average bioequivalence
Presenters:
 Robert SCHALL
Robert SCHALL
Professor, Statistical Consultation Unit, Department of Mathematical Statistics and Actuarial Science, University of the Free State, South Africa
 Co-author:
Co-author:
Divan BURGER
Senior Lecturer, Department of Statistics, University of Pretoria, South Africa
Summary of the course:
The short course presents robust methods for the assessment of both average and of scaled average bioequivalence, based on data from conventional cross-over studies and replicate design cross-over studies.
Initially, in an empirical study of a large number of average bioequivalence studies, the results of the
(i) classic,
(ii) conventional nonparametric and
(iii) Bayesian robust analyses are compared, and the need for robust analyses is discussed.
Thereafter, the classic and Bayesian robust methods are applied to a group of replicate design bioequivalence studies, and diagnostic plots and outlier diagnostics for such studies are presented. Robust analysis of replicate design bioequivalence data affects not only the estimation of the location parameters of the test and reference formulations, but also the within-reference scaling factor; a proposal is made for the appropriate handling of this matter.
The proposed Bayesian robust methodology is flexible with regard to study design (conventional cross-over, replicate design cross-over, parallel design) and with regard to the handling of outliers and skewness in the data.
Course Agenda
13:00 – 14:00 | 1st day Registration
 14:00 – 15:00 | 1st PART
14:00 – 15:00 | 1st PART
- Objectives
- Rationale
- Potential Need for Robust Methodology
- Approach: Bayesian Framework / t-Distribution
- Average Bioequivalence
- Statistical Model
- Types of Outlier
- Robust Methodology
![]() 15:00 – 15:20 | Coffee Break
15:00 – 15:20 | Coffee Break
 15:20 – 16:40 | 2nd PART
15:20 – 16:40 | 2nd PART
- Empirical Study
- Data Pool
- Need for Robust Methodology
- Degrees of Freedom
- Shift in Point and Interval Estimates
- Relative Confidence Interval Widths
- Method Comparison: Agreement between Methods
- Simulation study: Confidence Interval Coverage / Power
- Scaled Average Bioequivalence
- Statistical Model
- Types of Outlier
- Outlier plots
- Robust Methodology
![]() 16:40 – 17:00 | Coffee Break
16:40 – 17:00 | Coffee Break
 17:00 – 18:00 | 3rd PART
17:00 – 18:00 | 3rd PART
- Empirical Study
- Data Pool
- Need for Robust Methodology: Confidence Intervals and Degrees of Freedom
- Method Comparison: Agreement Between Methods
- Simulation study: Confidence interval coverage / Power
- Discussion / Summary
Discover more about the Forum:
SYMPOSIUM DAY PROGRAMME
Title of the short course:
- Robust methods for assessment of average and scaled average bioequivalence
Presenters:
 Robert SCHALL
Robert SCHALL
Professor, Statistical Consultation Unit, Department of Mathematical Statistics and Actuarial Science, University of the Free State, South Africa
 Co-author:
Co-author:
Divan BURGER
Senior Lecturer, Department of Statistics, University of Pretoria, South Africa
Summary of the course:
The short course presents robust methods for the assessment of both average and of scaled average bioequivalence, based on data from conventional cross-over studies and replicate design cross-over studies.
Initially, in an empirical study of a large number of average bioequivalence studies, the results of the
(i) classic,
(ii) conventional nonparametric and
(iii) Bayesian robust analyses are compared, and the need for robust analyses is discussed.
Thereafter, the classic and Bayesian robust methods are applied to a group of replicate design bioequivalence studies, and diagnostic plots and outlier diagnostics for such studies are presented. Robust analysis of replicate design bioequivalence data affects not only the estimation of the location parameters of the test and reference formulations, but also the within-reference scaling factor; a proposal is made for the appropriate handling of this matter.
The proposed Bayesian robust methodology is flexible with regard to study design (conventional cross-over, replicate design cross-over, parallel design) and with regard to the handling of outliers and skewness in the data.
COURSE AGENDA:
13:00 – 14:00 | 1st day Registration
 14:00 – 15:00 | 1st PART
14:00 – 15:00 | 1st PART
- Objectives
- Rationale
- Potential Need for Robust Methodology
- Approach: Bayesian Framework / t-Distribution
- Average Bioequivalence
- Statistical Model
- Types of Outlier
- Robust Methodology
![]() 15:00 – 15:30 | Coffee Break
15:00 – 15:30 | Coffee Break
 15:20 – 16:40 | 2nd PART
15:20 – 16:40 | 2nd PART
- Empirical Study
- Data Pool
- Need for Robust Methodology
- Degrees of Freedom
- Shift in Point and Interval Estimates
- Relative Confidence Interval Widths
- Method Comparison: Agreement between Methods
- Simulation study: Confidence Interval Coverage / Power
- Scaled Average Bioequivalence
- Statistical Model
- Types of Outlier
- Outlier plots
- Robust Methodology
![]() 16:40 – 17:00 | Coffee Break
16:40 – 17:00 | Coffee Break
 17:00 – 18:00 | 3rd PART
17:00 – 18:00 | 3rd PART
- Empirical Study
- Data Pool
- Need for Robust Methodology: Confidence Intervals and Degrees of Freedom
- Method Comparison: Agreement Between Methods
- Simulation study: Confidence interval coverage / Power
- Discussion / Summary
Regulatory keynote presentation (title is under finalization)
 Andrea LASLOP
Andrea LASLOP
Unit Head, Austrian Medicines and Medical Devices Agency (AGES), Austria
Title is under preparation
 Andreas BRANDT
Andreas BRANDT
Statistical assessor, Federal Institute for Drugs and Medical Devices (BfArM), Germany
Regulatory reflections on biosimilar development and statistical methods used at quality level
 Ina-Christine RONDAK
Ina-Christine RONDAK
Biostatistician (Seconded National Expert), European Medicines Agency (EMA)
CALL FOR ABSTRACT SUBMISSIONS
This year the Scientific Programme Committee plans to organize a Contributed Papers section and a Poster section within the Forum.
The organizers are pleased to invite authors to submit abstracts for poster and oral symposium presentations to be delivered at the 4th Annual Biosimilars Forum.
Email us your Abstract, participate at our event this year with your professional work!
![]()
COURSE DAY
 17 OCT, 2019
17 OCT, 2019
Professional short course in advanced biostatistics:
Robust methods for assessment of average and scaled average bioequivalence
|
|
Learn more: Abstract of the course
![]()
SYMPOSIUM DAY
 18 OCT, 2019
18 OCT, 2019
The symposium day of the forum consists of invited professional presentations, posters and round table sessions, the programme will be focusing on statistical and regulatory perspectives in biosimilar development. As in previous years, visitors can take part in the event with world-prominent subject matter experts from the fields of regulatory, academic and clinical research, who will be providing a detailed overview of current development issues of follow-on biologics and guidance for their handling.
|
|
Some topics from the programme of the Symposium day for presentations and discussions:
- New in this year: Special Regulatory Session on hot topics
an open, structured discussion within a round table framework with the participation of renowed regulatory expert speakers and panel members from several regulatory agencies around Europe.
- Scientific factors in biosimilar product development
- Recent developments and best practices in assessing biosimilarity
- Clinical study designs
- and much more with constructive professional exchanges about the current prespectives, development trends, burning issues and challenges of biosimilars
This page is frequently being updated, the detailed program and list of speakers will be available soon here, so please come back to discover the details or until then we recommend you read more our previous events!
Speakers of the 4th Annual Biosimilars Forum
Industry leaders, academic and clinical researchers, statisticians and regulatory representatives will gather together from around the world to share with each other their perspectives on the most important issues and challenges of biosimilar research. The programme of the event is under preparation, it will continue to expand until October with internationally renowned speakers from the biosimilars area. Some experts who have already accepted our invitation for this year’s Forum:

László ENDRÉNYI
Professor Emeritus of Pharmacology and Biostatistics, University of Toronto, Canada

Andrea LASLOP
Unit Head, Austrian Medicines and Medical Devices Agency (AGES), Austria

Robert SCHALL
Professor, Statistical Consultation Unit, Department of Mathematical Statistics and Actuarial Science, University of the Free State, South Africa

Divan BURGER
Senior Lecturer, Department of Statistics, University of Pretoria, South Africa

Arne RING
Professor of Statistics, University of the Free State, South Africa; Head of Biometrics and Statistical Programming, medac GmbH, Germany

Franz KÖNIG
Associate Professor, Section for Medical Statistics, Medical Unversity of Vienna, Austria

Andreas BRANDT
Statistical assessor, Federal Institute for Drugs and Medical Devices (BfArM), Germany

Stephan LEHR
Biostatistician, Austrian Medicines and Medical Devices Agency (AGES); former President of the Viennese Section of IBS, Austria

Ina-Christine RONDAK
Biostatistician, Seconded National Expert from Klinikum rechts der Isar of Technische Universität München to EMA, Germany

The list of speakers and special guests will continue to expand until October with internationally renowned experts

László ENDRÉNYI
Professor Emeritus of Pharmacology and Biostatistics, University of Toronto, Canada

Andrea LASLOP
Unit Head, Austrian Medicines and Medical Devices Agency (AGES), Austria

Robert SCHALL
Professor, Statistical Consultation Unit, Department of Mathematical Statistics and Actuarial Science, University of the Free State, South Africa

Divan BURGER
Senior Lecturer, Department of Statistics, University of Pretoria, South Africa

Arne RING
Professor of Statistics, University of the Free State, South Africa; Head of Biometrics and Statistical Programming, medac GmbH, Germany

Franz KÖNIG
Associate Professor, Section for Medical Statistics, Medical Unversity of Vienna, Austria

Stephan LEHR
Biostatistician, Austrian Medicines and Medical Devices Agency (AGES); former President of the Viennese Section of IBS, Austria

Ina-Christine RONDAK
Biostatistician, Seconded National Expert from Klinikum rechts der Isar of Technische Universität München to EMA, Germany
![]()
COURSE DAY
 17 OCT, 2019
17 OCT, 2019
Professional short course in advanced biostatistics:
Robust methods for assessment of average and scaled average bioequivalence
Presenters:
 Robert SCHALL
Robert SCHALL
Professor, Department of Mathematical Statistics and Actuarial Science, University of the Free State, South Africa
in cooperation with
 Divan BURGER
Divan BURGER
Senior Lecturer, Department of Statistics, University of Pretoria, South Africa
Learn more: Abstract of the course
![]()
SYMPOSIUM DAY
 18 OCT, 2019
18 OCT, 2019
with invited professional presentations, posters and round table session, will be focusing on statistical and regulatory perspectives in biosimilar development.
 Regulatory Keynote Speaker: Andrea LASLOP
Regulatory Keynote Speaker: Andrea LASLOPEuropean Regulatory Expert, Unit Head, Austrian Medicines and Medical Devices Agency, Austria
 Scientific Keynote Speaker: László ENDRÉNYI
Scientific Keynote Speaker: László ENDRÉNYIProfessor Emeritus of Pharmacology and Biostatistics, University of Toronto, Canada
The program for the second day will continue to expand until October with internationally renowned experts from the fields of regulatory, academic and clinical research who will be providing a detailed overview of current development issues of follow-on biologics and guidance for their handling. The agenda will be available soon here, until then we recommend you read more about our previous events!
Recent developments in biosimilars from the regulatory perspective
 Stephan LEHR
Stephan LEHR
Biostatistician, Austrian Medicines and Medical Devices Agency (AGES), Austria
Recent developments in biosimilars from the regulatory perspective
 Stephan LEHR
Stephan LEHR
Biostatistician, Austrian Medicines and Medical Devices Agency, Austria
Regulatory reflections on biosimilar development and statistical methods used at quality level
 Ina-Christine RONDAK
Ina-Christine RONDAK
Biostatistician (Seconded National Expert), European Medicines Agency
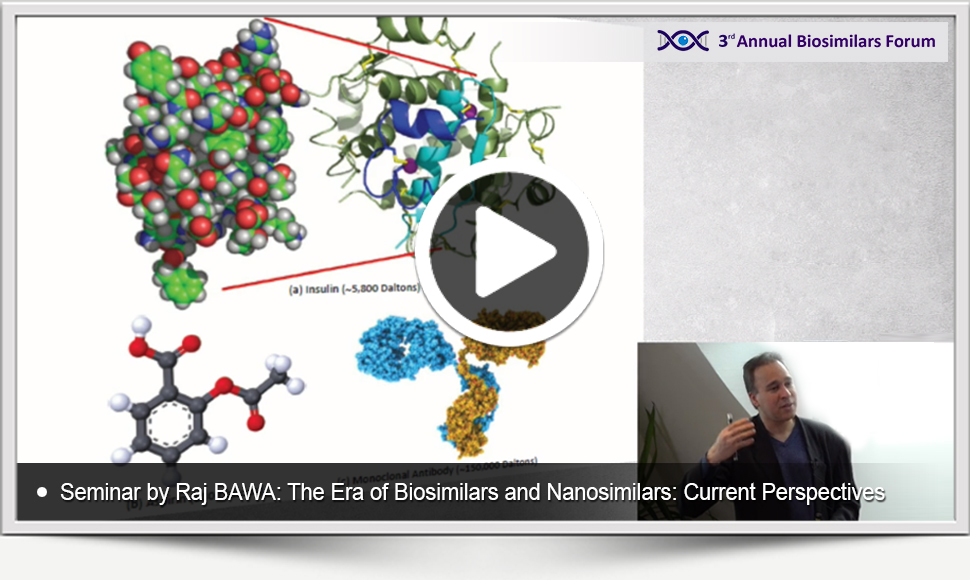
EXCLUSIVE VIDEO SEMINAR IN BIO- AND NANOSIMILARS
The presentation materials of the Seminar entitled The Era of Biosimilars and Nanosimilars: Current Perspectives delivered by Raj Bawa (Founding Director of American Society for Nanomedicine) at the 3rd Biosimilars Forum are available now to watch and download.
Sign up and get free access to the exclusive videos and materials of the seminar in bio- and nanosimilar development!
FRUITFUL
StrongCORE ScientificTM contribution
FRUITFUL
Maximize the value of your clinical development program with our flexible, fit-for-purpose consulting solution called StrongCore Scientific (SCS) and gain access to a multidisciplinary team of experts what is always uniquely composed, members are selected and involved in harmony with the special development needs …
EXCLUSIVE CONTENT – free video lecture series
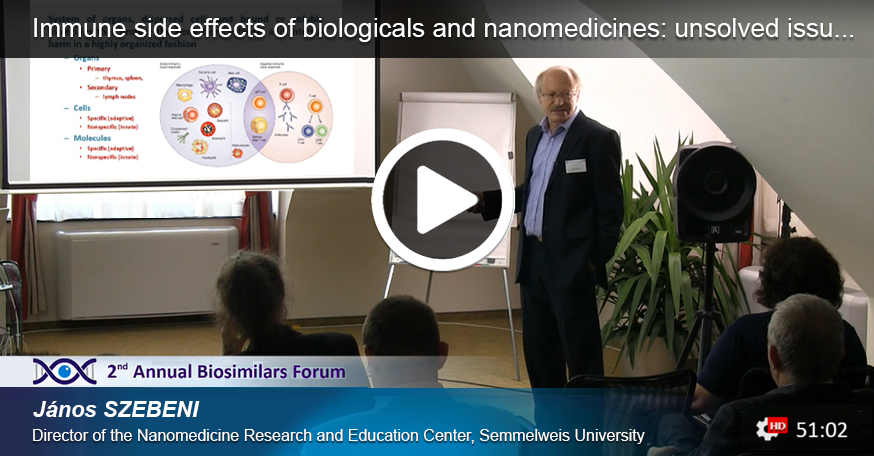
Sign up and watch all of the presentation videos recorded at the 2nd Biosimilars Forum for free.
This video lecture series includes professional courses & lectures relating to actual issues and challenges of follow-on biologics and guidance for their handling. The 8th video presentation about Immune side effects of biologicals and nanomedicines: unsolved issues in bio- and nanosimilar development lectured by János SZEBENI is available now.
SHORT AGENDA
At this 3-day series of events we will be focusing on the most actual issues and challenges in bio- and nanosimilar drug development with a strong focus on STATISTICAL and REGULATORY perspectives.
COURSE DAY
 25 OCT, 2018
25 OCT, 2018
with 2 professional course sessions on statistical and pharmacokinetical aspects of biosimilarity assessment, will be delivered by prominent biosimilar research expert scientists. Please, click here to reach the abstracts of the courses!
SYMPOSIUM DAY
 26 OCT, 2018
26 OCT, 2018
with invited professional presentations, posters and round table session, will be focusing on statistical and regulatory perspectives in bio- and nanosimilar development, presented by leading experts from the fields of regulatory, academic and clinical research.
SEMINAR DAY in Nanomedicines
 27 OCT, 2018
27 OCT, 2018
NEW IN 2018: the 3rd-day programme consists of professional Seminar sessions regarding current perspectives of bio- and nanosimilar drug development, will be lectured by special international experts from the field of nanotechnology-formulated drug research.
CALL FOR ABSTRACT SUBMISSIONS
This year the Scientific Programme Committee plans to organize a Contributed Papers section and a Poster section within the Forum.
The organizers are pleased to invite authors to submit abstracts for poster and oral symposium presentations to be delivered at the 3rd Annual Biosimilars Forum.
Email us your Abstract, participate at our 3rd event with your professional work!
SHORT AGENDA
This two-day series of events consists of a COURSE DAY with two professional lecture sessions (October 25th) and a SYMPOSIUM DAY (invited presentations, posters and round table session, scheduled for October 26th) comprising issues in biosimilar development with strong FOCUS ON Statistical and Regulatory Perspectives.
![]() 25th October, 2018 | COURSES with special lecturers
25th October, 2018 | COURSES with special lecturers
![]() 26th October, 2018 | SYMPOSIUM and ROUND TABLE
26th October, 2018 | SYMPOSIUM and ROUND TABLE
Tickets with flexible conditions are available now. Please send us your registration as soon as possible because places are limited in number! WBS and ISCB members can attend the 2nd day symposium for free.
![]() Registration deadline: 15th October, 2018
Registration deadline: 15th October, 2018